


产品性能与组成
该产品由一个316L手术级不锈钢球囊扩张支架和递送系统组成。支架推送系统球囊导管上嵌有二处不透射线标记,用以帮助进行支架放置。支架推送系统可与 0.035in(0.89mm)的导丝配合使用。支架推送系统球囊的最大充盈压力为12atm(1216kPa),可用于首次支架放置和支架置入后扩张。 环氧乙烷灭菌。一次性使用。
适用范围
该产品适用于治疗外周血管病变。
注册号:CFDA(I)20143463237
Compression resistance and conformability are crucial stent attributes that help improve vessel patency within tortuous vasculature.
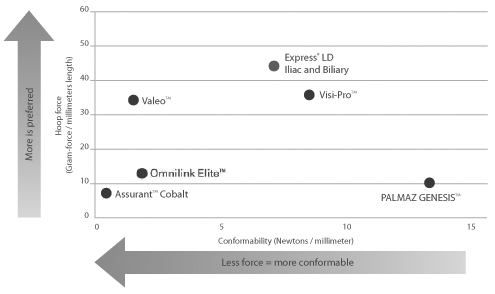
Compression Resistance measured as the force required to compress the fully expanded stent. Compression Resistance measured using a hoop force tester in 37° air. Conformability measured by torque required to achieve a constant curvature target as measured by angular deflection. Bench testing performed by Boston Scientific Corporation. Data on file. N = 3. Express LD tested: 8 x 37 mm. Visi-Pro tested: 8 x 37 mm. Palmaz Genesis tested: 8 x 39 mm. Omnilink Elite tested: 8 x 39 mm. Valeo tested: 8 x 36 mm. Assurant tested: 8 x 40 mm. Bench test results may not necessarily be indicative of clinical performance.
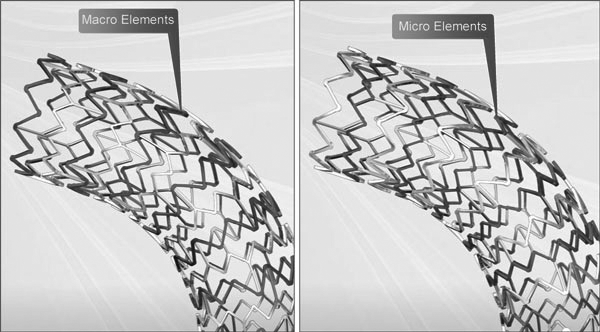
Micro™ Elements engineered to provide:

Macro™ Elements engineered for:

MELODIE Trial Summary
MELODIE is a prospective, multi-center, single arm study to obtain additional data on the safety and efficacy of the Express™ Vascular LD stent implantation in the treatment of stenosed or occlusive atherosclerotic disease (de novo or restenotic) in iliac arteries (common or external).
MELODIE Trial Objective
The objective was to demonstrate non-inferiority of the Express™ LD Stent for the treatment of atherosclerotic iliac artery lesions as compared to an objective performance criterion* (OPC), with a primary endpoint of mean percent luminal diameter loss at 6 months.
*Note: the OPC=15%, δ=5%.
Primary Endpoint
Definition
1Post MLD = Post-procedure minimum lumen diameter.
2FUP MLD = Follow-up minimum lumen diameter at 6 months.




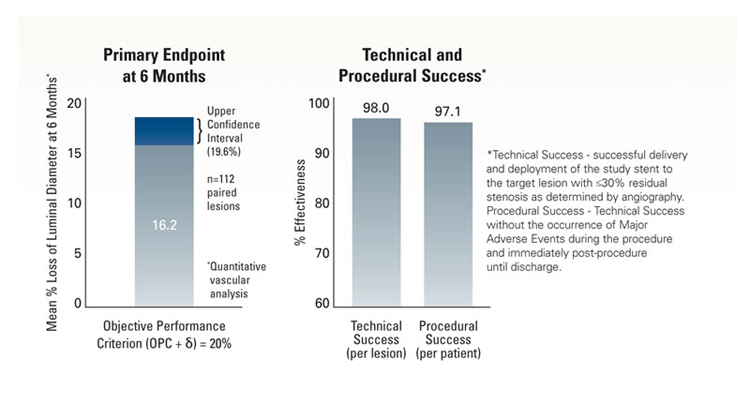
Outcome
24-Month Results
